chapter-5 ( class 10)
Periodic Classification of Elements
INTRO
*In the universe 115 elements have been discovered till today.
*Each of these elements possesses different properties.
*it is difficult to understand and use the properties of each elements at a time.
*hence attempts were made to discover ways to learn the properties of elements in a systematic order.
Doberenier's Triads :-
in 1829, more than 30 elements were known . Doberenier German scientist made some group of three elements each and called them TRIADS.
Characteristics:- atomic mass of second elements of a triad in nearly equal to the arithmetic mean of atomic masses of other two elements.
*Elements in triads have similar properties.
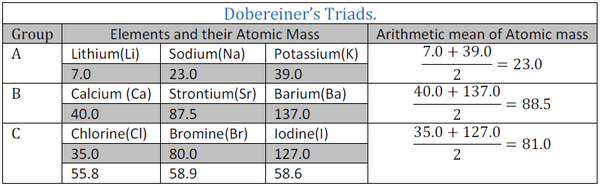
It is found that atomic mass of Sodium is arithmetic mean of first elements lithium and third element potassium and the properties of sodium were mean of properties of that of lithium and potassium.
Limitations
Dobereiner's idea of classification of elements into triads did not receive wide acceptance as he could arrange only q elements in triads form.
Newland's Law of Octaves :-
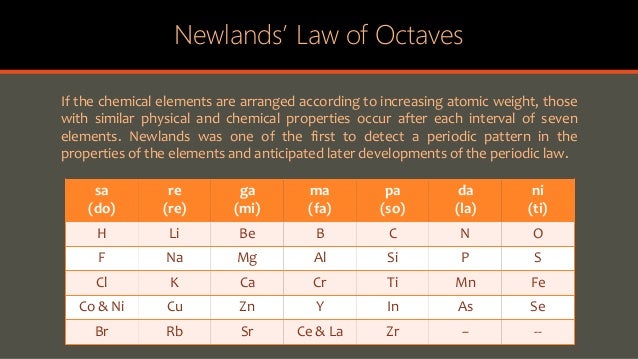
*Newland an English chemist in 1866 gave law of octaves.
*Till then 56 elements were known.
*Law of octaves say, 4 if elements are arranged by the increasing order of their atomic masses, property od every eighth elements. (starting from first elements) repeats 4.
Characteristics :-
*If contained the elements starting from hydrogen and ends at thorium.
*Properties of every eighth elements follow of that of first elements.
Limitations :-
*Similarly in properties of elements per the law was seen up to calcium only 20.
*Only 56 elements known at that time were talked about at that time around 1 elements was discovered every time. The elements to be discovered were not considered.
* At many places , 2 elements were placed in a single slot ( eg- Co & Ni )
*Placing of iron far away from cobalt and Ni , which have similar properties as Fe, could also not be explained.
Thankuh for the best explanation. It really helped me
ReplyDeleteNice explanation
ReplyDeleteToday
ReplyDelete